EPA Expands Temporary Process for Approval of Disinfectant Ingredients and Sources to Address Supply Chain Disruptions During COVID-19 Pandemic
On Tuesday, April 14, 2020, the U.S. Environmental Protection Agency (EPA) expanded its temporary amendment to the Pesticide Registration Notice 98-10, superseding the one issued on March 30, 2020. The aim of the temporary amendment is to help ensure that disinfectant products effective against SARS-CoV-2 remain available to the country during the COVID-19 pandemic.
Before registrants take advantage of this policy, it is important to understand that after the termination date for this temporary amendment, registrants will not be able to release for shipment formulations produced under the conditions allowed by this temporary amendment without first submitting a new notification, as appropriate, or an application to amend the confidential statement of formula (CSF) and receiving EPA approval of that amendment. EPA will provide seven days’ notice prior to terminating this temporary amendment.
Scope of the Temporary Amendment
- Product Chemistry Notifications for Commodity Active Ingredients
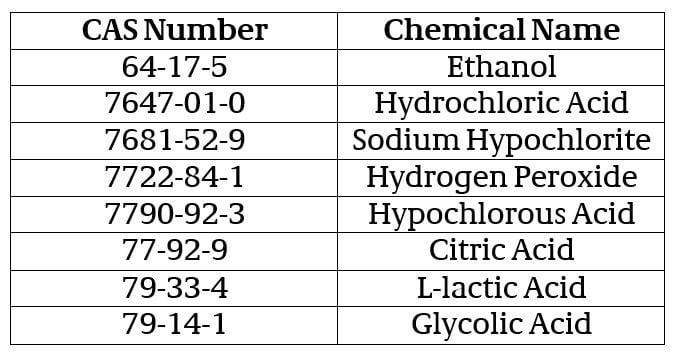
Keeping with the March 30th modification, EPA is continuing to allow manufacturers of registered EPA disinfectants to obtain certain “commodity” active ingredients from any source of suppliers without checking with the agency first. The temporary policy applies to the following list of active ingredients, provided that the resulting formulation is chemically similar to the current source (i.e., meets the criteria given in Section III.A. of PR Notice 98-10):
- Notifications to Substitute Sources of Similar Inert Ingredients or Non-Commodity Active Ingredients
Registrants can also substitute the source of inert ingredients and Non-Commodity Active Ingredients, so long as the composition of the ingredients are similar. Here, the term “similar” means that the active or inert ingredient obtained from the source will have the same CAS number as well as the same purity. If a registrant is substituting the source of inert ingredients, it must also provide EPA the composition information from the inert ingredient supplier. - Adjusting Inert Ingredients when Sources of Commodity Active Ingredients or Substitute Registered Sources are not similar
EPA recognizes that it may not be possible to substitute commodity sources of active ingredients or substitute registered sources of active ingredients with similar alternate sources. In these situations, it may be necessary to adjust the amount of inert ingredients to ensure the nominal concentration of the active ingredient in the product does not change. So long as the nominal concentration of active ingredients in the product remains the same, EPA will now permit registrants to adjust the amount of water in a product without the requiring confirmatory efficacy data. - Notification to add an EPA-Registered Establishment
When there are no other changes to the formulation other than what is permitted under this temporary amendment, EPA will permit registrants to add an EPA-registered establishment for formulations that have a registered source of active ingredient.
Procedure for Submission of Notifications Under this Temporary Amendment
Registrants who wish to take advantage of this temporary policy must first submit an application via the CDX Portal along with a cover letter to EPA that includes the following information:
- In the subject line, state: “Notification per TEMPORARY AMENDMENT TO PR NOTICE 98-10 for EPA Registration No. XXXXXX and [insert product name];”
- Include the active ingredient; and
- The following statement:
“[Name of Registrant] is submitting this notification consistent with the provisions of PR Notice 98-10 and [insert section(s)] of the Temporary Amendment to PR Notice 98-10 dated April 14, 2020, and no other changes have been made to the confidential statement of formula or labeling of this product. I confirm that the ingredients statement of this label remains truthful. I understand that it is a violation of 18 U.S.C. Sec. 1001 to willfully make any false statement to EPA. I further understand that if this self-certification is not consistent with the terms of PR Notice 98-10, the Temporary Amendment 98-10 dated April 14, 2020, and 40 CFR 152.46, this product may be in violation of FIFRA and I may be subject to enforcement actions and penalties under sections 12 and 14 of FIFRA.”
Once received, email the CDX tracking number (CDX 2020_XXXXXXX) to the Product Manager of the registered product.
Registrants may begin to distribute or sell a product modified under the temporary policy once EPA receives the notification.
For more information on EPA’s temporary policies, please contact Kimberly DalSanto or a member of Taft’s Environmental team.
Please visit our COVID-19 Toolkit for all of Taft’s updates on the coronavirus.
In This Article
You May Also Like
EPA Withdraws Endangerment Finding, Rescinds Vehicle GHG Emission Standards EPA Tightens NOx Standards for New Stationary Combustion Turbines